PALYNZIQ® (pegvaliase-pqpz) Injection is a daily at-home injectable treatment.
Here are a few things to know about how you will take PALYNZIQ:
- You will give yourself daily injections just under the skin (subcutaneously) while at home
- PALYNZIQ comes in prefilled syringes that can be injected in a few steps
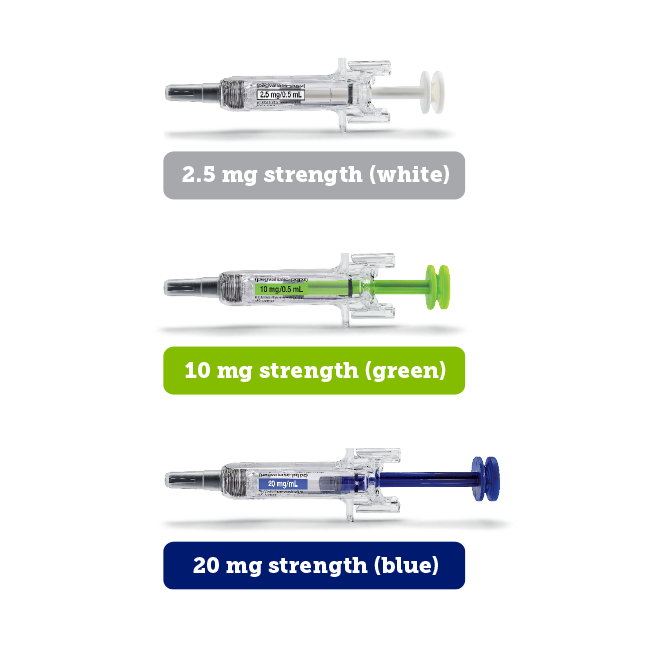
PALYNZIQ is available in 3 dosage strengths:

 Hesitant about self-injection?* Learn about the educational and support options available to you
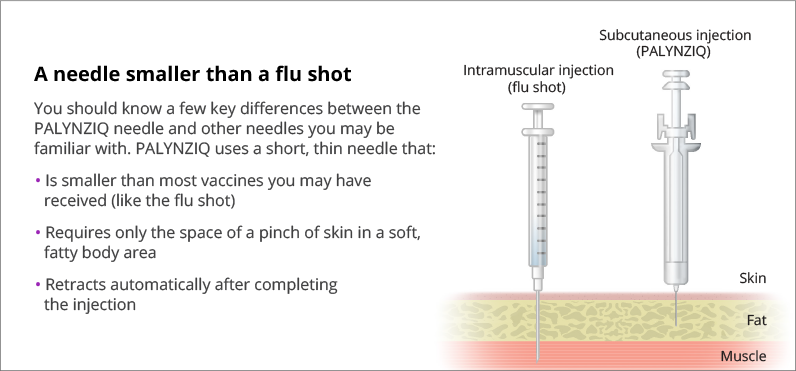
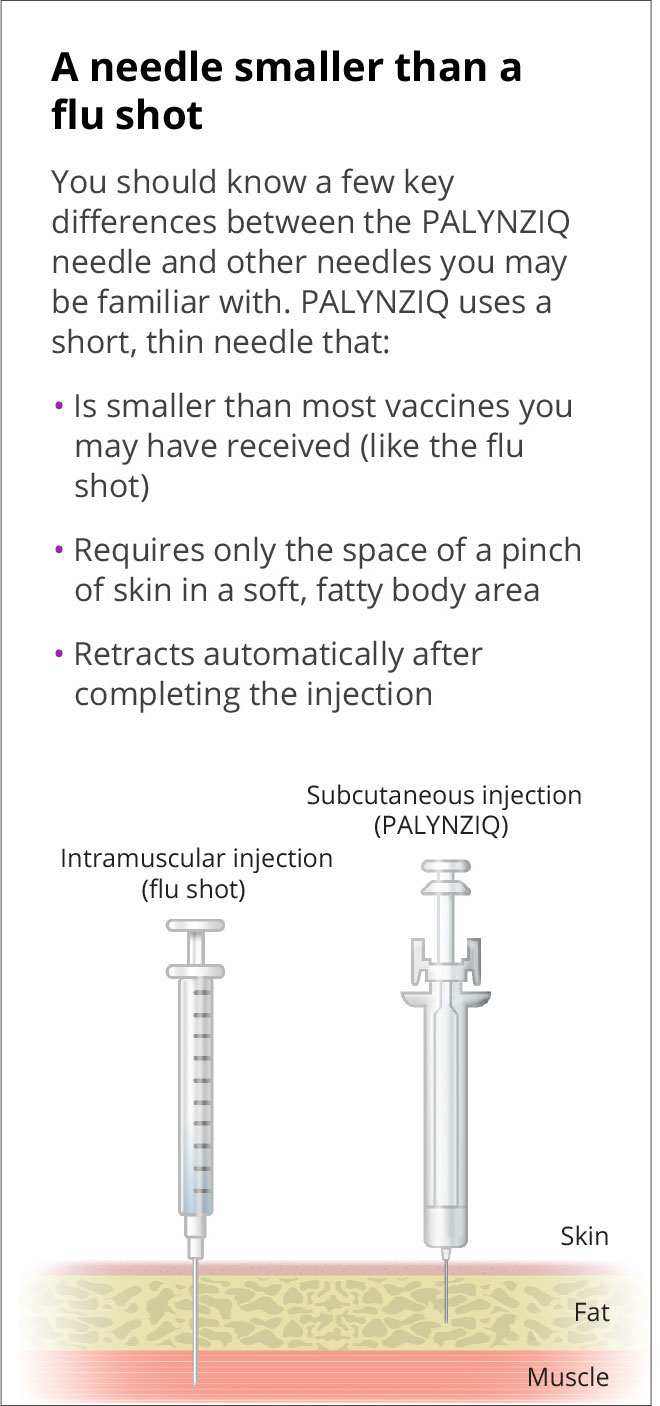
Hesitant about self-injection?* Learn about the educational and support options available to youPALYNZIQ uses a subcutaneous injection, which is inserted into the fatty tissue just under the layer of skin. It does not go as far into the body as an intramuscular injection.
Finding a dose to meet your treatment goals
Your clinic team will slowly adjust the dose of your PALYNZIQ to find a dose that works for you.
You will start by injecting PALYNZIQ once a week. Then, your healthcare provider will adjust your dose based on how you’re responding to therapy.
Here is an example of how dosing with PALYNZIQ may look:
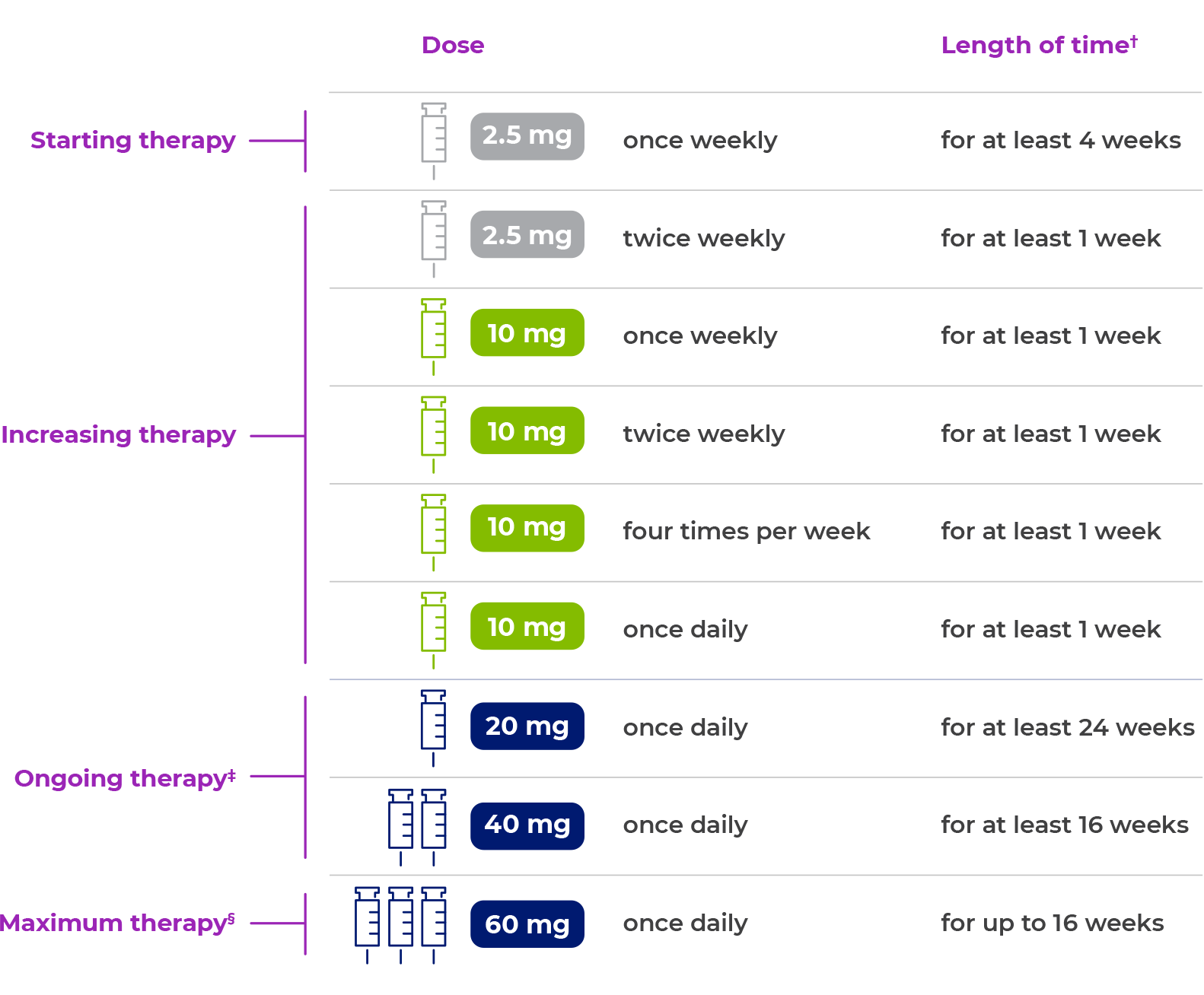
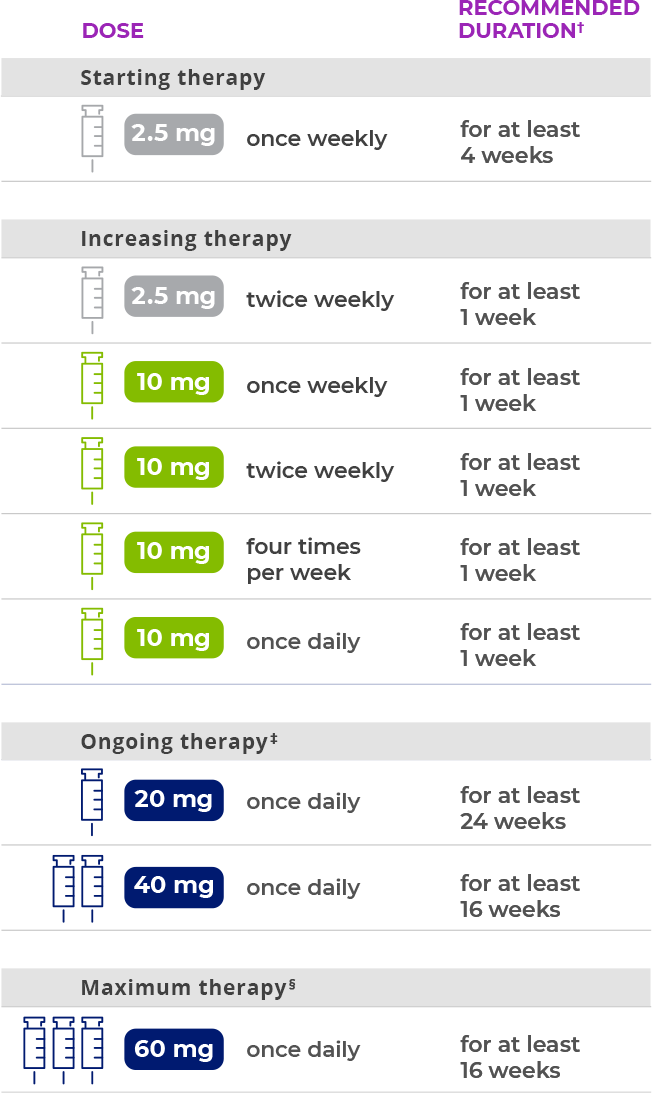
Additional time may be required prior to each dosage escalation based on patient tolerability.
Individualize treatment to the lowest effective and tolerated dosage. Consider increasing to 40 mg once daily in patients who have not achieved a response with 20 mg once daily continuous treatment for at least 24 weeks. Consider increasing to a maximum of 60 mg once daily in patients who have not achieved a response with 40 mg once daily continuous treatment for at least 16 weeks.
PALYNZIQ should be discontinued if patients have not achieved an adequate response after 16 weeks of continuous treatment at the maximum dosage of 60 mg once daily.
Everyone responds differently to PALYNZIQ and finding the right dose for you will take time.
The reason PALYNZIQ is introduced slowly and over time is to allow your immune system to get used to the medication.
Videos to help with your PALYNZIQ journey
PALYNZIQ Injection Training
This video demonstrates injection instruction that can be used as a guide to help you with PALYNZIQ. This information should not take the place of your healthcare provider’s instructions or direction related to your medical condition or your treatment.
Overcoming Injection Fear
Ease your fear of needles and injections with a brief overview of the product education and support that you’ll receive with PALYNZIQ.
Before injecting PALYNZIQ, talk to your healthcare provider right away if you cannot or will not use auto-injectable epinephrine to treat a severe allergic reaction. If you are pregnant or plan to become pregnant while taking PALYNZIQ, talk to your healthcare provider to discuss the risks and benefits of taking PALYNZIQ during pregnancy to you and your unborn baby. If you are breastfeeding or plan to breastfeed, talk to your healthcare provider to discuss the risks and benefits of taking PALYNZIQ while breastfeeding for you and your baby.
You’re not alone—when you start taking PALYNZIQ, your clinic team will:
- Show you how to properly inject PALYNZIQ
- Be present as you give yourself your first injection
- Confirm your ability to self-administer PALYNZIQ
- Teach you about the signs and symptoms of anaphylaxis, and what to do if you have anaphylaxis
- Instruct you on how to use auto-injectable epinephrine in case it’s needed due to anaphylaxis
- You must carry your auto-injectable epinephrine with you at all times
Side effects can occur with PALYNZIQ
PALYNZIQ can cause a severe allergic reaction (anaphylaxis) that may be life threatening and can happen at any time during treatment with PALYNZIQ.
Your clinic team will train you to recognize the signs and symptoms so you’ll be ready and well-prepared to manage this reaction, should it occur. These include:
- Fainting (passing out)
- Dizziness or lightheadedness
- Sudden confusion
- Trouble breathing or wheezing
- Chest discomfort or chest tightness
- Fast heart rate
- Swelling of your face, lips, eyes, or tongue
- Throat tightness
- Flushed skin
- Skin rash, itching, or raised bumps on skin
- Nausea, vomiting, or diarrhea
- Losing control of urine or stools
10% of patients (29 of 285) experienced anaphylaxis in clinical studies

- Of the 10% of patients who experienced anaphylaxis in PALYNZIQ studies, 72% of them were able to return to treatment with PALYNZIQ under the supervision of their clinic team
- Of those patients who returned to treatment, the majority (71%) did not experience anaphylaxis again
- All anaphylaxis episodes in the study ended without further health consequences for the patients
For your safety, your clinic team will prescribe auto-injectable epinephrine. Keep the auto-injectable epinephrine with you at all times during treatment with PALYNZIQ.
Common side effects when patients in clinical studies were starting (induction) and increasing (titration) their PALYNZIQ dose (N=285)
Your clinic team will start you on a low dose and slowly increase your dose so that your immune system can adjust to PALYNZIQ.
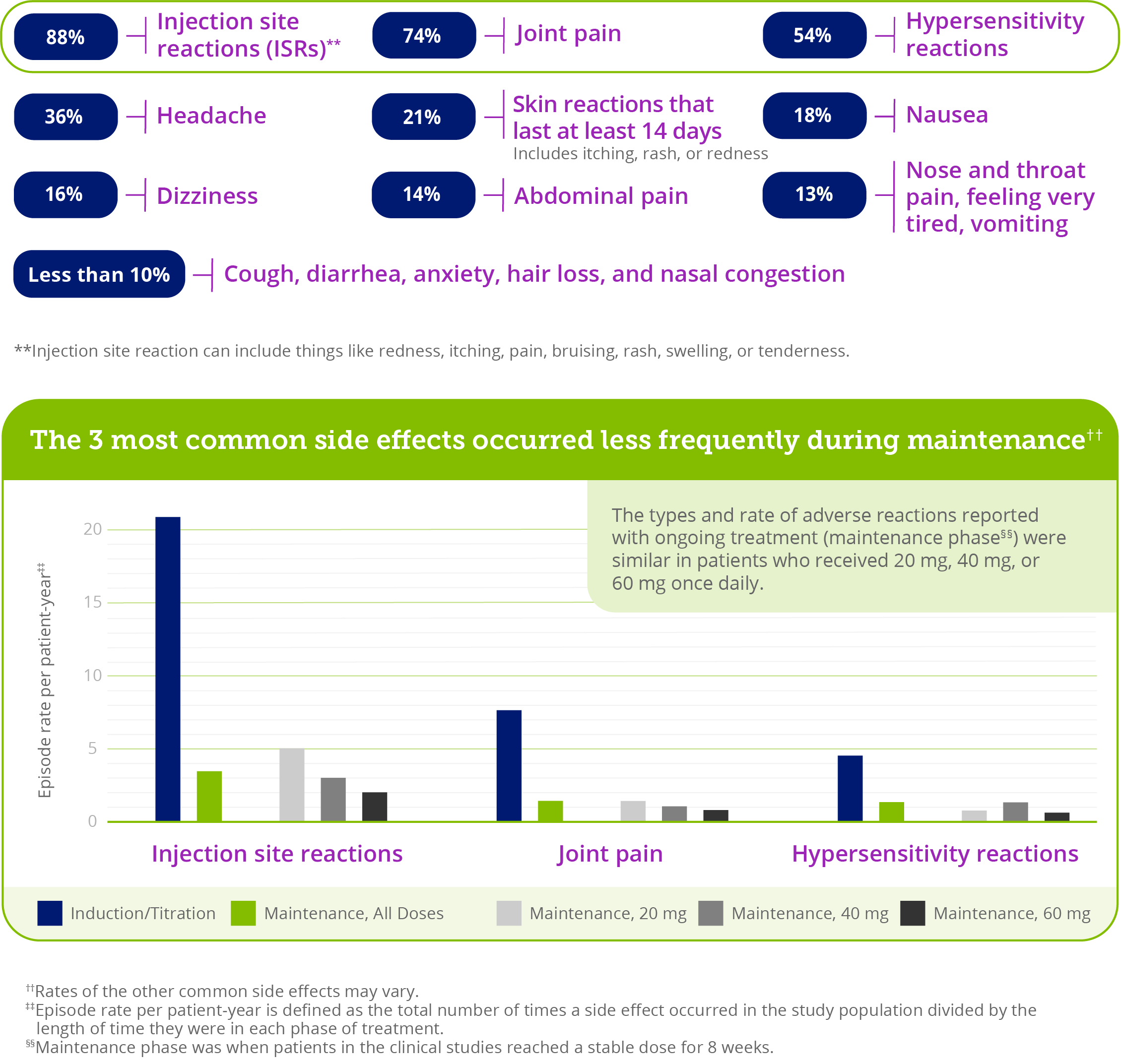

- While most patients in clinical studies experienced a side effect while taking PALYNZIQ, only 15% of people had to stop treatment due to side effects
- During maintenance (ongoing), common side effects were similar to those seen when starting therapy and while increasing therapy
- It’s also important to know that while most patients in clinical studies experienced a side effect while taking PALYNZIQ, only 15% of people stopped treatment due to side effects
Most side effects seen with PALYNZIQ in clinical studies were considered mild to moderate and generally decreased over time—many of which can be managed.
View a video about anaphylaxis
About Anaphylaxis
This video will help you learn more about the potential risk of anaphylaxis with PALYNZIQ and how your care team will ensure you know how to respond if it ever were to occur.
The PALYNZIQ Risk Evaluation and Mitigation Strategy (REMS)
You can get PALYNZIQ only through the PALYNZIQ REMS program. The purpose of the program is to ensure that you have been fully informed of the risk of anaphylaxis associated with taking PALYNZIQ, and that you understand this risk before you start treatment. Your clinic team can tell you more about PALYNZIQ REMS and help you enroll.
Your journey with PALYNZIQ
If you and your clinic team decide that PALYNZIQ® (pegvaliase-pqpz) Injection is right for you, here are the steps you will follow to get started:


Step 1
Discuss PALYNZIQ with your clinic team.
Your healthcare provider will take you through the PALYNZIQ Risk Evaluation and Mitigation Strategy (REMS), which will help you understand:
- The risks associated with PALYNZIQ, including a severe allergic reaction (anaphylaxis)
- The need for auto-injectable epinephrine
Afterwards, you’ll fill out a patient authorization form that allows BioMarin RareConnections™ to contact you about support services.
Step 2
Get support throughout your treatment journey.
In person or over the phone, BioMarin Clinical Coordinators will work closely with you to provide injection training and ongoing reinforcement of how to properly inject PALYNZIQ. They will also provide ongoing product education and support throughout your treatment journey.


Step 3
Learn more from a BioMarin RareConnections™ Case Manager.
They will work with your clinic team and support you by confirming insurance benefits, identifying financial assistance options, and coordinating shipment of medication. Your Case Manager will provide information about financial assistance options, including the PALYNZIQ Co-Pay Assistance Program for eligible patients.


Step 4
Receive PALYNZIQ in the mail.
PALYNZIQ is only available from pharmacies that participate in the PALYNZIQ REMS.
The pharmacy will:
- Verify that you have received and filled a prescription for auto-injectable epinephrine
- Call you to schedule a shipment of PALYNZIQ that will come right to your home


Step 5
Administer your first injection under the supervision of a healthcare professional.
A trained healthcare professional will be there to teach you how to inject PALYNZIQ, answer any questions, help you with dosage adjustments, and monitor your blood Phe levels.


Step 6
Stay on therapy with the help of ongoing support.
BioMarin is committed to providing personalized product education and support services to help you start and stay on therapy. You’ll have access to comprehensive resources and individualized support throughout your treatment with PALYNZIQ.