As with all therapeutic proteins, there is potential for immunogenicity1

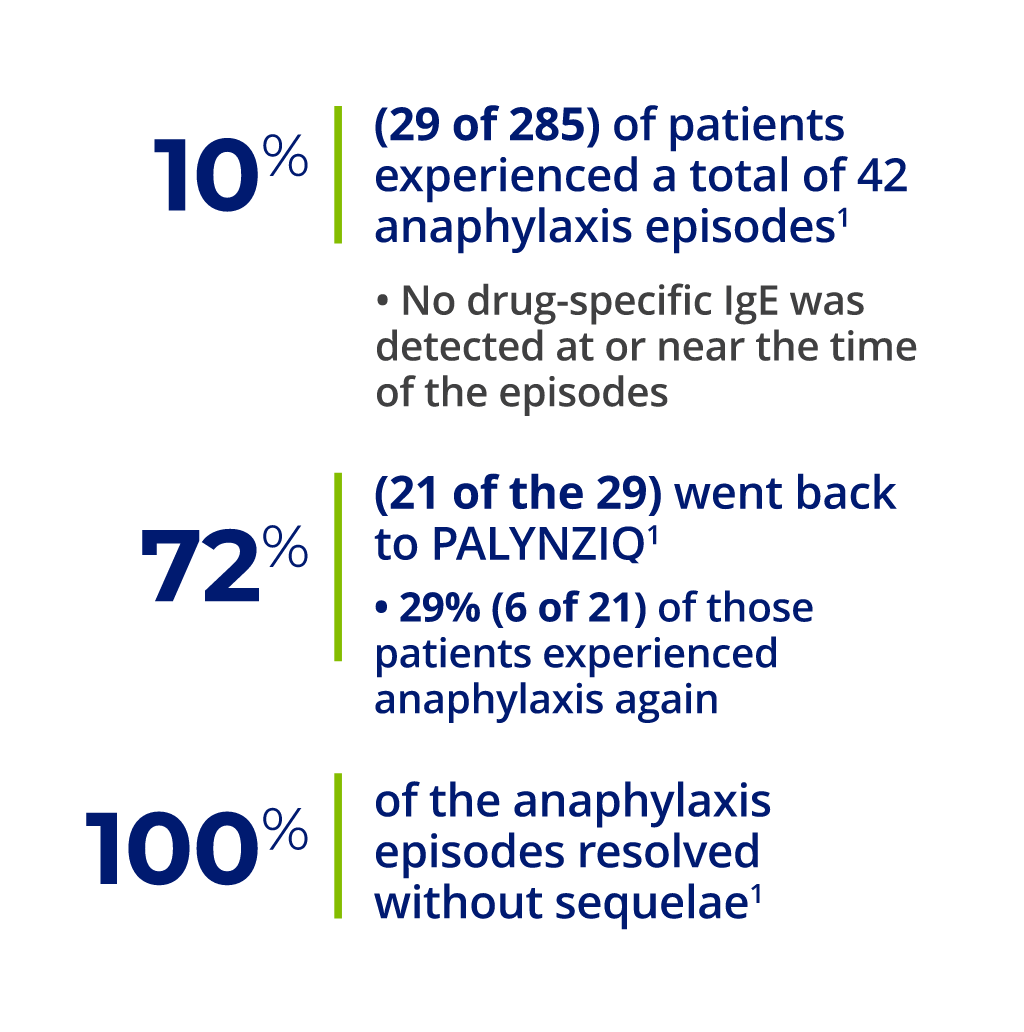
a 27 of 29 patients who had anaphylaxis were tested for anti–pegvaliase-pqpz IgE antibodies, which recognize the PEGylated protein product. Of those 27 patients, 26 tested negative. The one patient who screened positive for anti–pegvaliase-pqpz IgE had insufficient sample to confirm IgE positivity. This patient tested negative for anti–pegvaliase-pqpz IgE at routine visits before and after the anaphylaxis episode.1
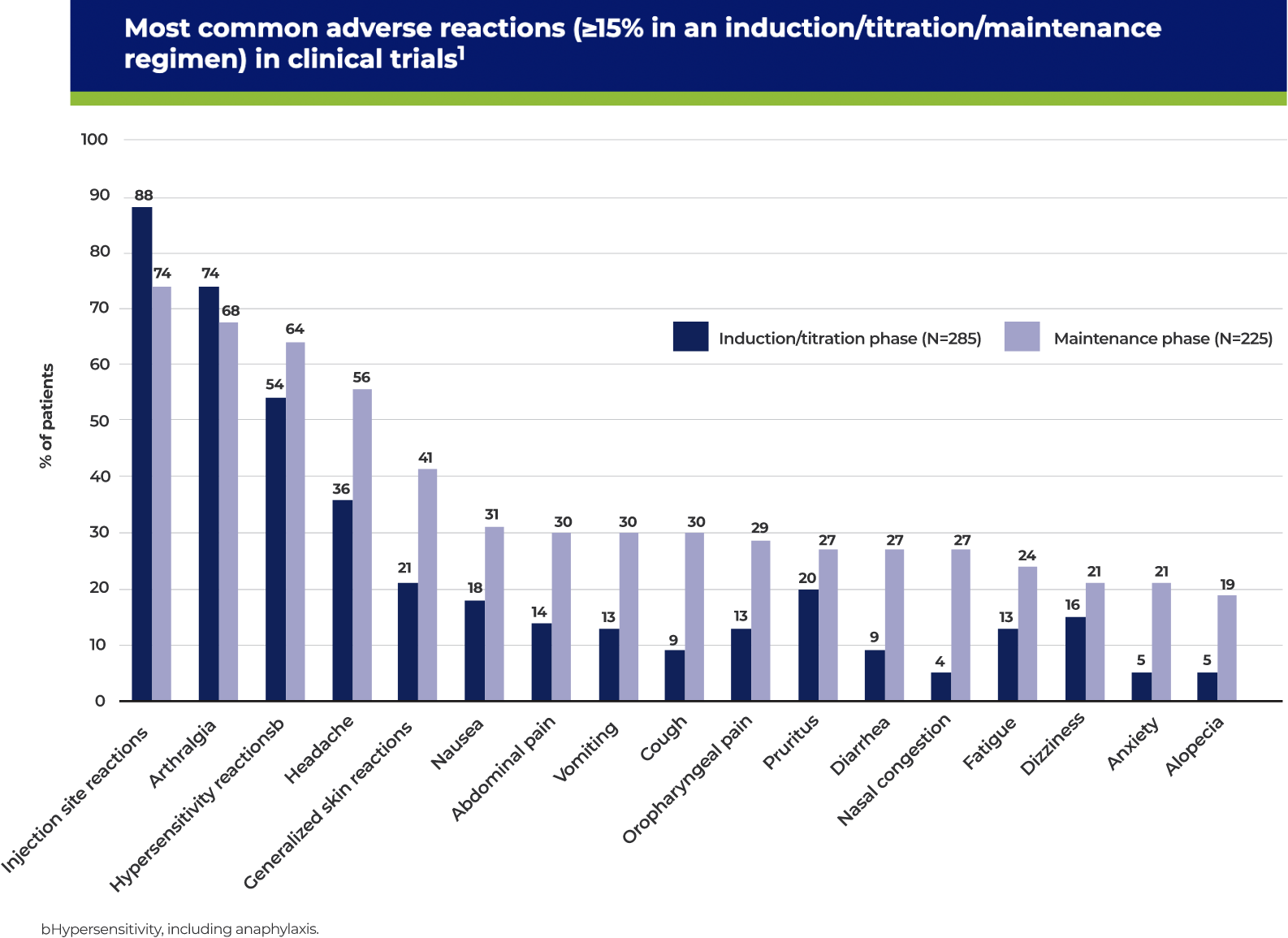
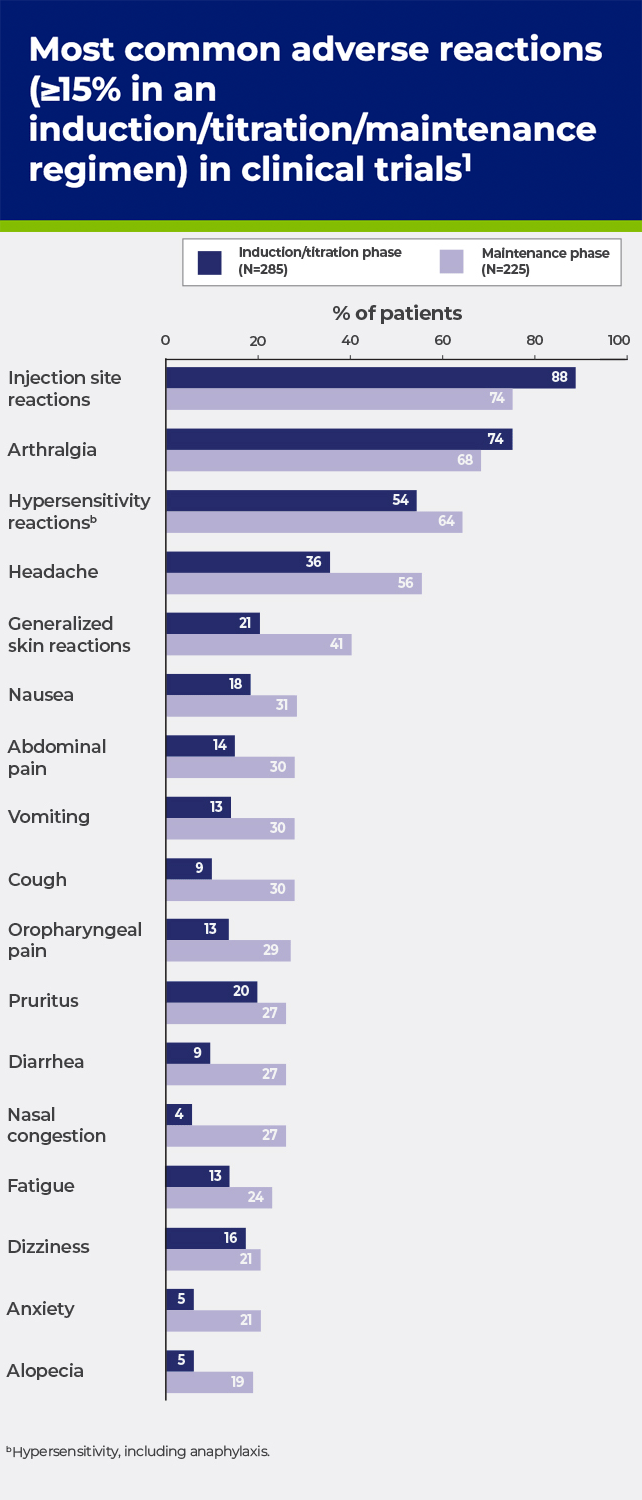
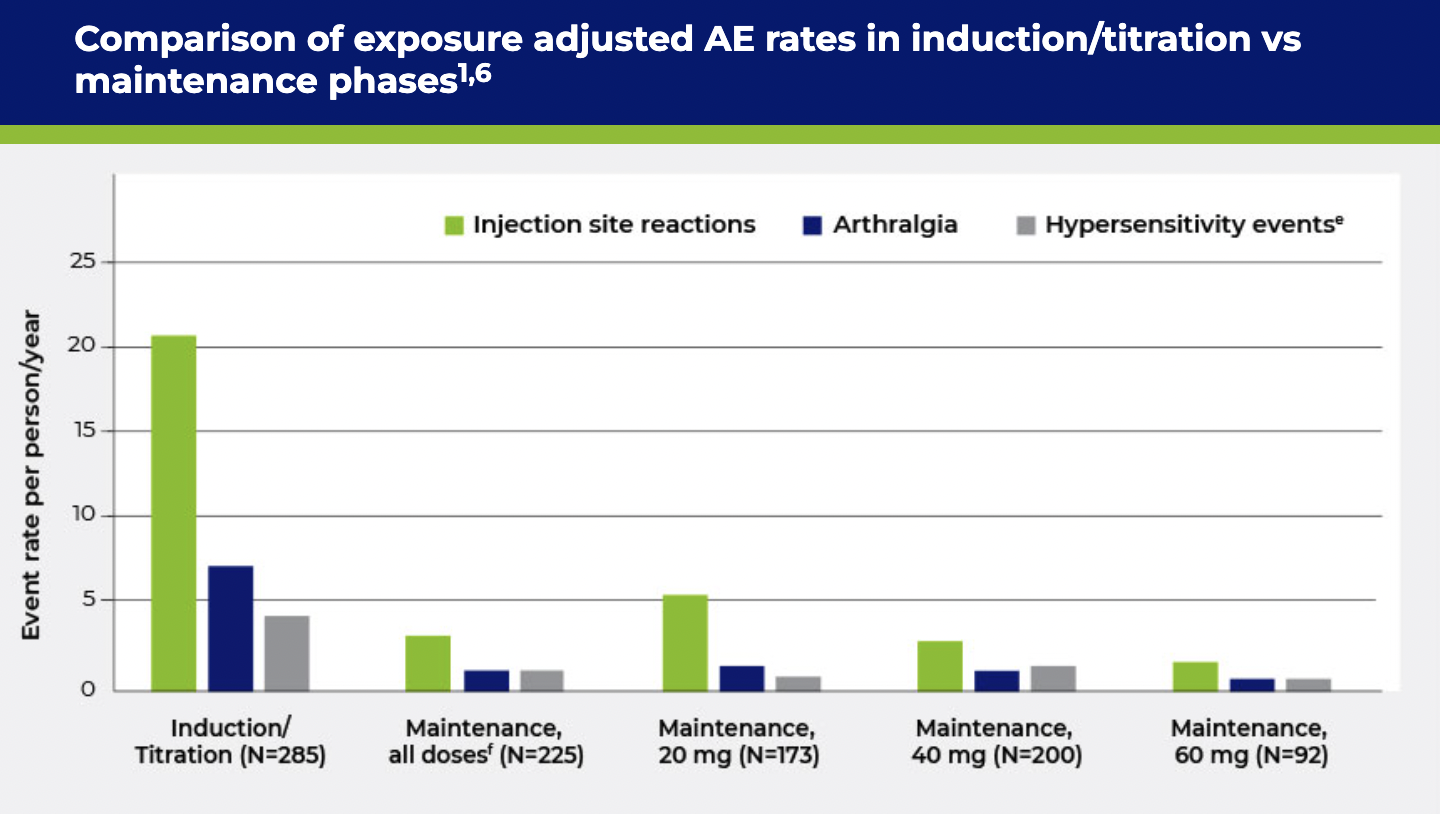
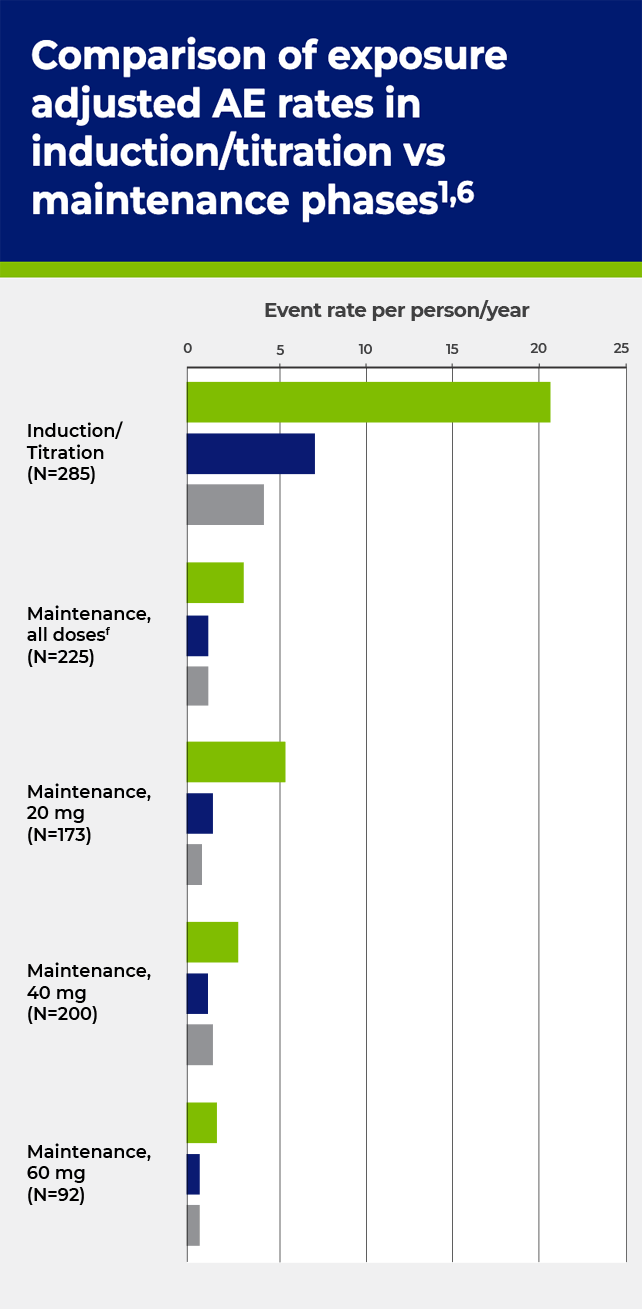
cHypersensitivity, including anaphylaxis.
dMaintenance phase defined as when subjects reached stable dose for 8 weeks.
eHypersensitivity, including anaphylaxis.
fMaintenance, all doses includes patients on placebo and PALYNZIQ doses <20 mg.
Premedication may be considered, based on individual patient tolerability, prior to each dose of PALYNZIQ. Most frequently administered H1- and H2-receptor antagonists and antipyretics during PRISM-2 g,h,i,j


gPatients (n=215) may have been prescribed premedications in combination, and/or may have switched between different premedication regimens during the study period.
hThe rates of use of these medications in this population only partially reflects their use as premedications; these medications may also have been used to treat other conditions, such as seasonal allergies, headaches, etc.
iDuring PRISM-2, premedication was administered 2-3 hours prior to PALYNZIQ.
jPlease note: the FDA has advised a voluntary recall of ranitidine made by many manufacturers, due to concerns over purity. Consider substituting a different H2 blocker.
BOXED WARNING: RISK OF ANAPHYLAXIS
WARNINGS AND PRECAUTIONS
Anaphylaxis
Other Hypersensitivity Reactions
ADVERSE REACTIONS
Blood Phenylalanine Monitoring and Diet
DRUG INTERACTIONS
Effect of PALYNZIQ on Other PEGylated Products
USE IN SPECIFIC POPULATIONS
Pregnancy and Lactation
Pediatric Use
Geriatric Use
You are encouraged to report suspected adverse reactions to BioMarin at 1-866-906-6100, or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, with Boxed Warning for risk of anaphylaxis, and Medication Guide here.
INDICATION
PALYNZIQ is a phenylalanine (Phe)-metabolizing enzyme indicated to reduce blood Phe concentrations in adult patients with phenylketonuria who have uncontrolled blood Phe concentrations greater than 600 micromol/L on existing management.