Discover long-term efficacy and safety data out to 3 years
Personalize your patients’ dosing regimens to help them achieve blood Phe levels ≤360 µmol/L

Access all the necessary forms and resources to get your patients started on PALYNZIQ
A Phe-restricted diet* is not required in conjunction with PALYNZIQ. The majority of patients were not on a Phe-restricted diet* prior to and during the study.1
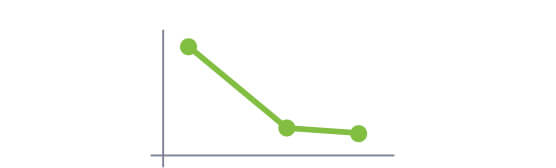
A cross-sectional analysis showed that 66% (57 of 86) of patients had blood Phe levels ≤360 µmol/L at 24 months, and 50% (43 of 86) of the patients had blood Phe levels ≤120 µmol/L at 24 months.1
The majority of patients (89/118) achieved blood Phe levels ≤360 µmol/L, with 50% of them achieving this level by 10 months in the study.1,3
Rates of hypersensitivity reactions decreased over time from induction/titration to maintenance phases, despite increasing dose.1
*A Phe-restricted diet is defined as >75% of protein intake from medical food.1
BOXED WARNING: RISK OF ANAPHYLAXIS
WARNINGS AND PRECAUTIONS
Anaphylaxis
Other Hypersensitivity Reactions
ADVERSE REACTIONS
Blood Phenylalanine Monitoring and Diet
DRUG INTERACTIONS
Effect of PALYNZIQ on Other PEGylated Products
USE IN SPECIFIC POPULATIONS
Pregnancy and Lactation
Pediatric Use
Geriatric Use
You are encouraged to report suspected adverse reactions to BioMarin at 1-866-906-6100, or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, with Boxed Warning for risk of anaphylaxis, and Medication Guide here.
INDICATION
PALYNZIQ is a phenylalanine (Phe)-metabolizing enzyme indicated to reduce blood Phe concentrations in adult patients with phenylketonuria who have uncontrolled blood Phe concentrations greater than 600 micromol/L on existing management.